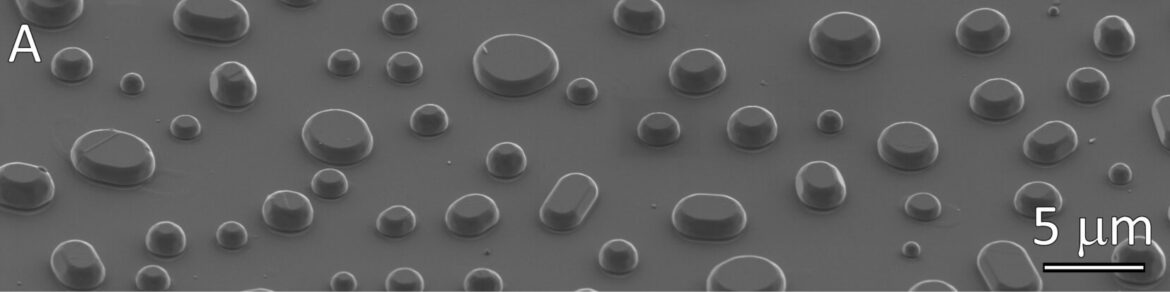
The group had a rich history of work on the topic of wetting; mostly at metal-ceramic interfaces. While initial studies focused on the wetting of ceramics by metals in the liquid state, a significant effort was made to study wetting of solid-solid interfaces. This is conducted using Winterbottom analysis of “dewetted” and equilibrated films to determine the energy of solid-solid interfaces. Data from wetting is often applied to understand processing of ceramic matrix composites and ceramic-metal and ceramic-ceramic joins. A significant effort includes the correlation of solute adsorption to interfaces with the decrease in excess interface energy and atomistic structure (from advanced microscopy). In recent years we have expanded our work on solute adsorption to grain boundaries, and experimental correlation of the influence of solute excess on the mechanism and kinetics of grain boundary mobility. Finally, in-depth electron microscopy of the interfaces and grain is conducted. Major microscopy challenges include quantification of interfacial chemical excess, interface atomistic structure, the measurement of extremely low solubility limits at high temperatures, and characterization of the equilibrium crystal shape of materials using correlative microscopy.
Wetting
Solid-solid interface energy measurements are based on Winterbottom analysis, using cross-section TEM of site-specific samples prepared using FIB. Again, the goal of these studies is to obtain a correlation of solid interface energy to processing temperature, gas partial pressure, chemistry, and crystallographic orientation. Systems under study currently include various ceramic phases in contact with ZrO2, ZrC, SiC, and of course Al2O3.
Composites and Ceramic Microstructures
Data from the wetting experiments are important in their own right, but are also used for designing processing schemes for metal-ceramic joints and composites, which is the second topic related to metal-ceramic interfaces which is studied in Kaplan’s group. Kaplan’s group has developed a unique method for producing nanocomposites. The process is based on the infiltration of metallic salts into a fired (green) ceramic preform. During sintering, the gas phase in the sintering furnace is controlled to promote reduction of the salts, to form nano-sized metallic particles within the open pores of the ceramic body, which is then sintered to full density. By controlling the partial pressure of gases in the sintering atmosphere (e.g. O2, N2), the particles can be maintained in the metallic state, or react with the matrix to form ceramic particles. The mechanical response of the composite, in particular to abrasive wear, is of current interest.
In parallel and related to the ceramic matrix, the group explores the influence of adsorbing solutes on changing the rate of grain boundary mobility. This includes doping the ceramic matrix at concentrations confirmed to be below the high temperature solubility limit, and the influence of external fields in modifying the local distribution of adsorbing cations, anions, and charge compensating defects.
Adsorption, Rapid Heating, and Densification
Since 2024 Kaplan’s group is exploring the possible existence of metastable grain boundary states, and the influence of these metastable states on grain boundary diffusion. This is done by using extremely rapid heating (>2400°C/min) and/or adsorption. Rapid heating is obtained using a new furnace system constructed in Kaplan’s group. The implications of increased grain boundary diffusion is the ability to sinter ceramics in minutes instead of hours, at processing temperatures which are lower than standard.
Current Research Projects
Disconnections at grain boundaries in alumina, and their modification by adsorbing dopants;
High temperature solubility limits of key dopants and impurities in ceramics;
The modification of surface diffusion kinetics of ceramics via surface adsorption;
The influence of external fields on grain boundary mobility;
The measurement of the equilibrium crystal shape of ceramic materials as a function of solute adsorption;
The correlation between atomistic structure/chemistry and interface thermodynamics.